Bats carry a lot of viruses that make humans and other mammals very sick, but don’t make them sick. Here’s what we currently understand about why that is.
It’s a weird aspect of bat biology, viruses that make other mammals very, very ill don’t appear to make them sick.
It’s not that all viruses don’t make all bats sick. Bats can get sick from lyssaviruses – which includes the rabies virus – and a lesser known group of viruses called arenaviruses.
But what about all the rest?
Ebola, Hendra virus, Nipah virus and SARS-CoV-2 can all be found in bats, but they don’t appear to get sick when they’re infected.
The number of viruses that do make bats sick seems to be fewer than what we see with other mammals.
Burnet Institute virologist Dr Joshua Hayward explains what we currently understand about why most viruses don’t make bats sick.

There are two things that set bats apart from other mammalian species, according to what Dr Hayward and the other members of the Retroviral Biology and Antivirals research team led by Professor Gilda Tachedjian, along with their collaborators at the Australian Centre for Disease Preparedness at the CSIRO, have learnt from their research.
“The first is that they’ve got an increased tolerance for viral infection,” he said.
“And the second is that they have unique adaptations in their immune system that helps them manage their viral infections in a manner different to humans and other mammals.”
Both these characteristics are due to the one thing that makes them the most different from all other mammals – their ability to fly.
“The fact that they've evolved to fly has directly led to this distinction that we see in the way that they tolerate their viral infections.”
Flight is very, very energetically expensive, Dr Hayward said.
“Animals that fly have very fast metabolisms, and that generates inflammation inside their cells.”

Inflammation is a complex response to harmful stimuli, but it’s a double-edged sword: while essential for helping us fight infection, it can also cause cellular damage.
“Where bats are concerned, they've evolved to reduce their inflammatory response as a necessary outcome of having evolved to fly,” Dr Hayward said.
"They fly so they have fast metabolisms, so they would be generating a lot of inflammation in their cells, and that would be bad for them.”
Dr Joshua Hayward, Burnet Institute
“But they've turned the knob on their inflammation dial all the way down. And this is important because viral infections also cause inflammation.”
Having a reduced inflammatory response means viruses don’t stimulate the same kind of inflammation in bats that they would in humans and other mammals, meaning they can tolerate viral infection inside their cells without inflammation making things worse.
“What we propose is that because they have this environment in their cells that's a bit more hospitable for viruses, they've had to evolve also additional ways of managing those viral infections which might become persistent in bats.”
And there are changes in other parts of their immune systems that enable them to manage viral infections too.
How are our immune systems different to those of bats?
We last shared a common ancestor with bats around 55 to 60 million years ago, Dr Hayward said, so it’s not surprising that our immune systems are significantly different.
There are over 1,400 species of bats in the world, accounting for more than 20 per cent of all mammalian species.
Some of those bat species would have split from each other almost as far back as we diverged from bats.
“So when we talk about bat immune systems we’re talking about lots of different changes, some of which might be present in one species but not in another,” Dr Hayward said.
But the big immune system differences we see between bats and humans are their limited inflammatory response to infection, and the expanded and diversified repertoire of antiviral proteins that they have.
See also: Bats live with dozens of nasty viruses — can studying them help stop pandemics?
What do antiviral proteins do?
If you think of viruses as being like tiny biological machines whose job is to replicate and spread, antiviral proteins are like tiny spanners you throw into the machine to stop it working.
These antiviral proteins can work in lots of ways, Dr Hayward said.
“They can stop viruses from entering a host cell, they can stop viruses from making new copies of themselves, or they can stop viruses from leaving the cell to infect new ones.”
Dr Hayward’s research has been looking at a particular antiviral protein called tetherin.
We’ve known about tetherin since it was discovered about 15 years ago, Dr Hayward said. And we’ve learnt since then that almost all mammals have a single tetherin gene.
In humans that single tetherin gene makes two different tetherin proteins that are very similar to each other
"But when we looked at bats, we found that different bats produced all sorts of different tetherins.”
Dr Joshua Hayward, Burnet Institute
For example, in a recent study published by the Retroviral Biology and Antivirals group at Burnet, in collaboration with the CSIRO, they focused on fruit bats and vesper bats.

Fruit bats also have a single tetherin gene, but they use that gene to make multiple, different types of tetherin that appear to have quite different functions from one another, whereas vesper bats have at least five tetherin genes, each one producing multiple different tetherin proteins.
“So we've seen that a gene that makes one type of protein in humans has expanded and diversified in bats. And what this is telling us is that maybe tetherin has new jobs, or it has the same job but it does it differently, or maybe it targets a broader range of viruses than human tetherin.”
If you look at kidney cells under a microscope producing either human tetherins (left) or bat tetherins from the Australian black flying fox (right) glowing green in the pictures below, you can see what while human tetherins go to the surface of the cells, bat tetherins go both to the surface and are also all through the inside of the cells.
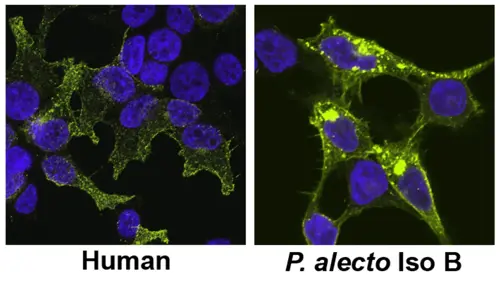
“That’s really fascinating,” Dr Hayward said, “We want to know more about what these bat tetherins are doing differently and why.”
"Bats have a fundamentally different relationship with viruses than humans or other mammals do. And they've been learning how to manage their viruses differently for over 50 million years.”
Dr Joshua Hayward, Burnet Institute
Does this make bats a threat to humans?
The straight-up answer is no, Dr Hayward said.
“Bats are not a threat, although some of the viruses that they carry are. It’s human and societal behaviour that’s driving spillover of viruses from these bats.”
The destruction of bats’ natural habitat and the loss of their sources of food in the wild is what’s pushing them to overlap their living space with ours.
“We are the drivers of viral spillover from bats into humans,” Dr Hayward said.
But there are ways we can mitigate this viral threat.
“If we reduce the pressure on bats that drives them into our habitats, then we reduce the likelihood of viral spillover into humans,” Dr Hayward said.
And ensure that bats continue to play their really vital roles in our ecosystem, including pollinating our native trees and helping to keep insect populations down.
Beyond that we could look at vaccinating intermediate hosts.

For example, we have a Hendra vaccine for horses because the pathway for Hendra virus to come from bats into humans is to come through other animals such as horses first.
We also need to support animal virus research, Dr Hayward said.
"Knowing as much as we can about the viruses that are being carried by bats will allow more front-footed approaches when spillover does happen.”
Dr Joshua Hayward, Burnet Institute
If we weren’t pre-armed with almost two decades of research into SARS-CoV-1 when SARS-CoV-2 came onto the scene, we would have had a much harder time developing vaccines and treatments to combat COVID-19, Dr Hayward said.
“We're trying to understand the unique ways that different mammals such as bats have of fighting viruses. Having that understanding of these unique mechanisms or these unique weaknesses that viruses might have, that's what can stimulate the development of new interventions like drugs and medicines.”
Read more of Burnet's latest news
Disease Elimination
Bat viruses and antiviral immunity
Globally we have witnessed the increasing emergence of deadly viruses transmitting from animals to humans including the current SARS-CoV-2 pandemic. Accordingly, the need to take a proactive response to better understand emerging viruses and their animal hosts has never been so great. Bats are an important reservoir of various viruses, including new and emerging viruses such as Ebola and SARS-associated coronaviruses, which are deadly to humans and other mammals but do not cause visible signs of illness in bats. Investigating unique features of the immune system of bats will shed light on how they tolerate viral infections, potentially informing novel antiviral strategies in humans and other animals.

